 weather modification report
weather modification reportI was kicked off of CTC for standing upto the moderator. View my latest chemtrail/weather modification discussion at the home page. The 6 month plan is to post all of my old weather discussions to make a case for the USAF's weather modification project.
Abstract:
The long white trails left behind by jet aircraft in the sky are not contrails but chemical trails. I will use a simple greenhouse model and create a mock experiment to prove or disprove, thermodynamically whether or not chemical trails are the sole reason for the rain to dissipate and the impending drought. The results of the experiment conclude that chemical trails are not the sole reason for the rain to dissipate and for the drought.
Introduction:
The United States is currently in a critical drought that started in September 2001, in the same month of the World Trade Center attacks. Since that time there has been an enormous increase of chemical trail activity reported by citizens across the United States. At the same time the amount of commercial aircraft flights has decreased significantly. On pages 7 and 8 there are time lapse photos of a contrail passing underneath cirrus clouds, and leaving nothing behind, that is an example of a contrail as the condensed water evaporates. The trails shown in the photo section of this paper show otherwise. The trails are long and wide, very uncharacteristic considering the moisture left behind by a contrail is limited. In San Jose, California, Tony Diconza took this picture on April 11,2002, from his back yard, of a chemical trail spraying device breaking from the plane and going in a different direction. The trail left behind the plane is clearly a chemical trail, and if it was a contrail the plane would have met its fate because that would be the engine going in a different direction. There were no reports of a plane crash that day in California.
Pictures of Contrail through cirrus clouds:



Picture of Exploding Sprayer

The chemical trails are used for 3D imaging of the terrain using RF. The Navy has the capability to shoot RF at the ionosphere, and obtain a surface scan of the ocean several hundred miles away. The problem is, the RF signals do not perform very well over the terrain. Engineering past this, the Navy had to find a way to “duct” the RF to obtain good 3D images of the terrian. Barium Salts provide this ducting, and allow for imaging of buildings with Satellite overlay. This technology gives our troops the best and up to date information on the battle field.
The majority of precipiation producing fronts that become sprayed with chemical trails produce less then 0.25” rainfall over a given area. This correlates to roughly 3 cm/sec in vertical rise in the air column over a square meter. It is my theory from my observation since January of 2002, that the chemical trails are causing the drought through heating of the atmosphere between the trail and the base of the precipiation producing clouds. I will conduct a simple theoretical experiment to examine if these chemical trails create a large enough greenhouse effect between the trail and the clouds, to cause a large enough reduction in rainfall to be responsible for the drought.
Theory:
The theory for this experiment is obsverations across the country of NEXRAD radar sites, and matching Satilette photos to the radar, to see where the chemical trails are affecting precipitation. I will give a synopsys of three events, Feb 28,2002, over Lake Erie, March 8, 2002, over the Mid-Atlantic, and April 11,2002, over the Southeast.
The first event I captured is over Lake Erie on Feburary 28,2002, the Satilette photo shows lake effect snows over a very warm Lake Erie. A NASA color Satilette photo captures the Chemical Trails in action prefectly. Over Southwestern Lake Erie, Eastern Michigan and Western Ohio, a layer of Chemical trails is visible over top the snow pack. Two lake effect snow bands are forming from the winds across the Lake Erie. The remarkible thing is, the snowbands start to intensify where the Chemical Trails end, right in the middle of Lake Erie. The temperatures of Lake Erie would have supported cloud formation only a few hunderd yards away from the lake shore. This is not the case.
The second event I captured was March 8, 2002. A powerful storm in the planes with its surface cold front across the Mid-West was expected to cross the Mid-Atlantic states the next day on the 9th. The night before the precipiation arrived, chemical trails where being laid from Georgia, through Maine, with the exception of Virginia, Maryland, Delaware , Southeastern PA, and Southern New Jeresey. The next afternoon, some rain developed, but only in the regions described above, and dissipated in about 3 hours as it traveled to the northeast, where the chemical trails where laid the evening before. The rainfall totals for this event ranged from 0.05” to 0.18”. The expected rainfall was near 0.25”.
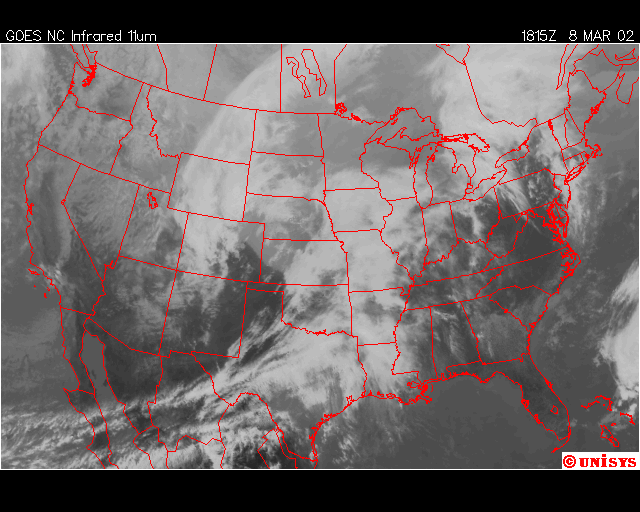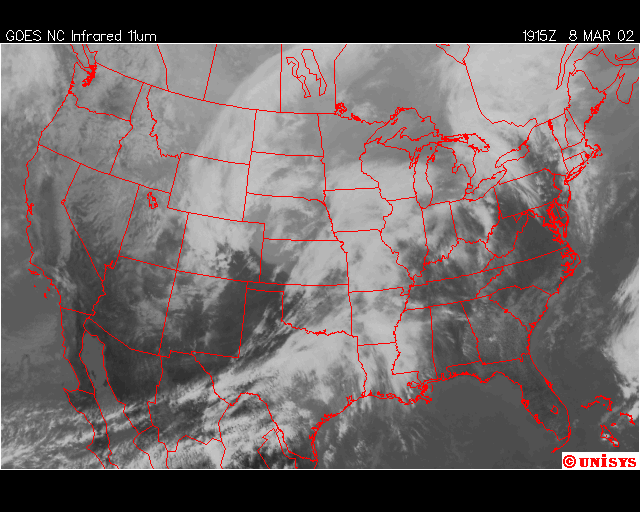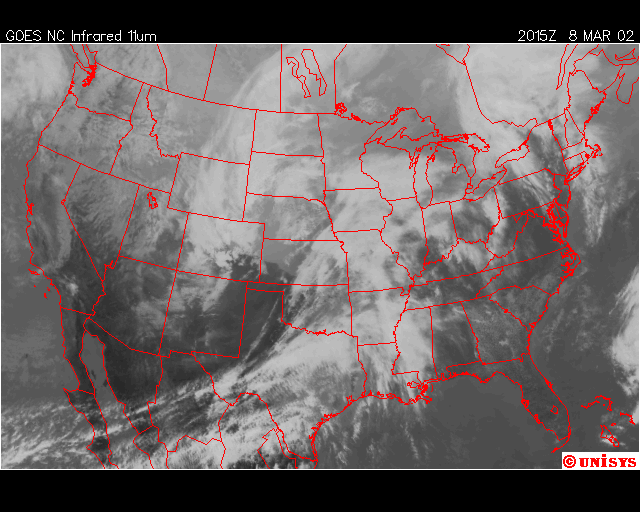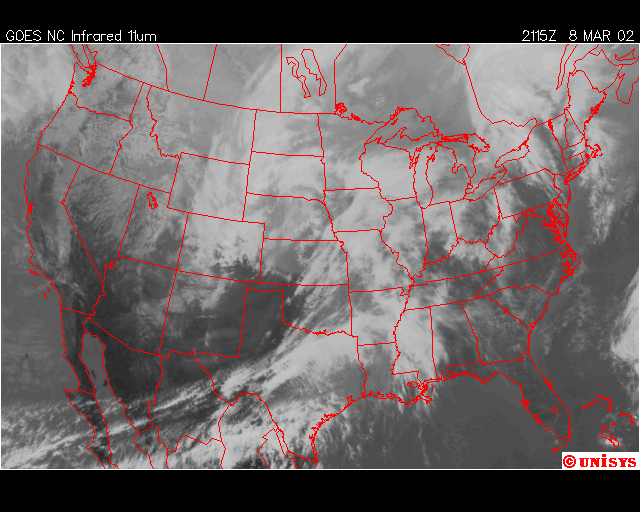
Finally, over Southeastern United States on the afternoon April 11,2002, an upper level low near Lousiana is setting off numerous showers and thunderstorms across the region. Everywhere from Eastern Texas to South Carolina recieved rain from this event that day,except Southwern Georgia. In Southwestern Georiga, the Chemical Trails where evident on the Satliette pictures. The area that was sprayed with Chemical Trails did not recieve any rain from this event for April 11,2002.
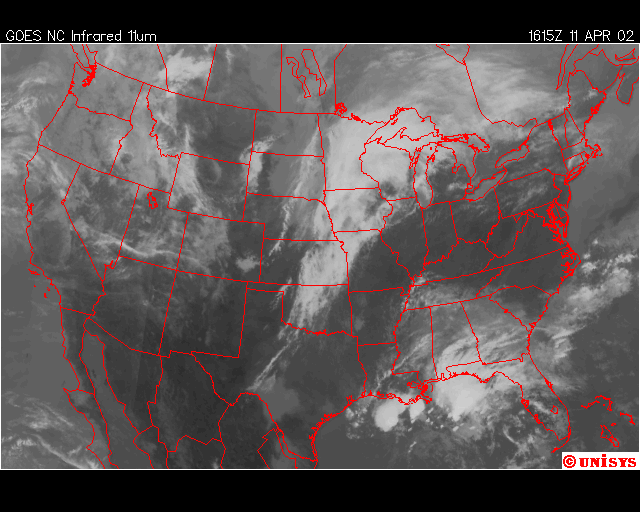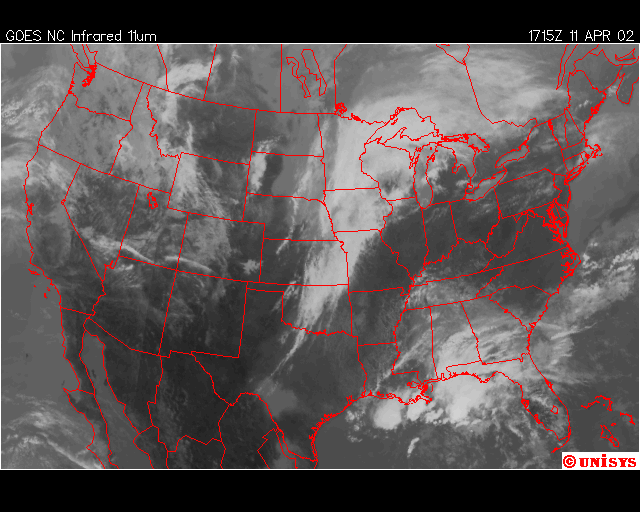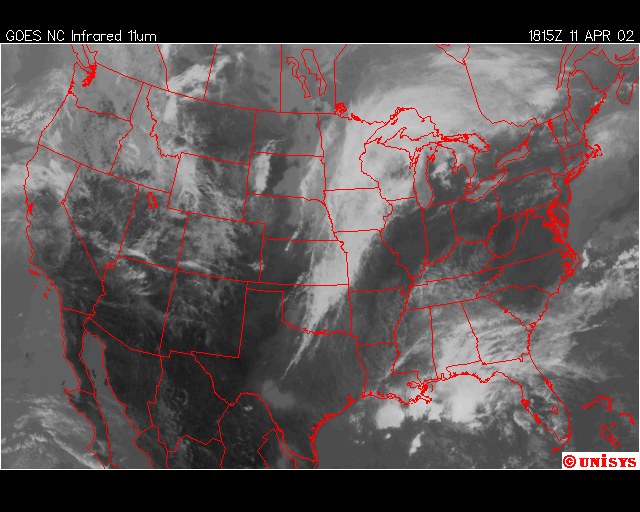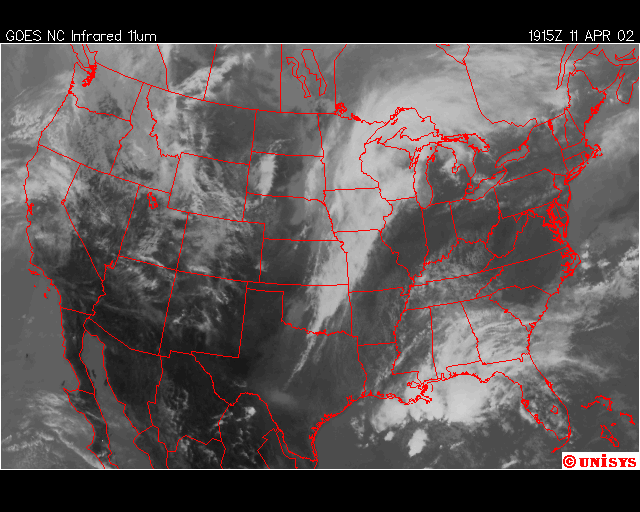
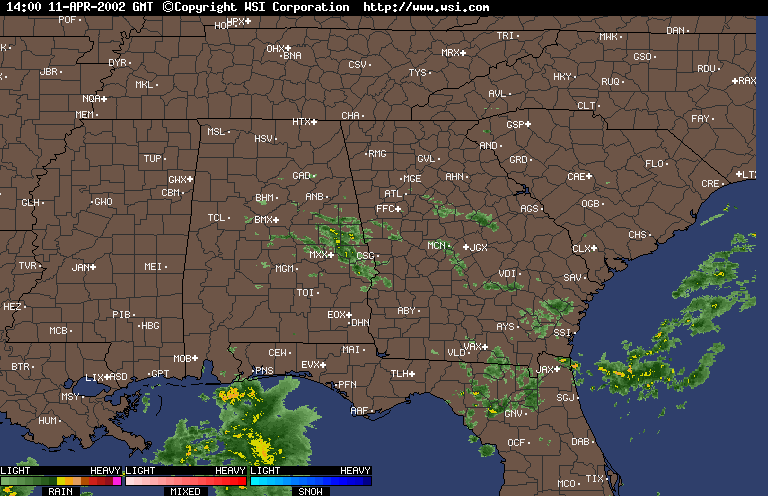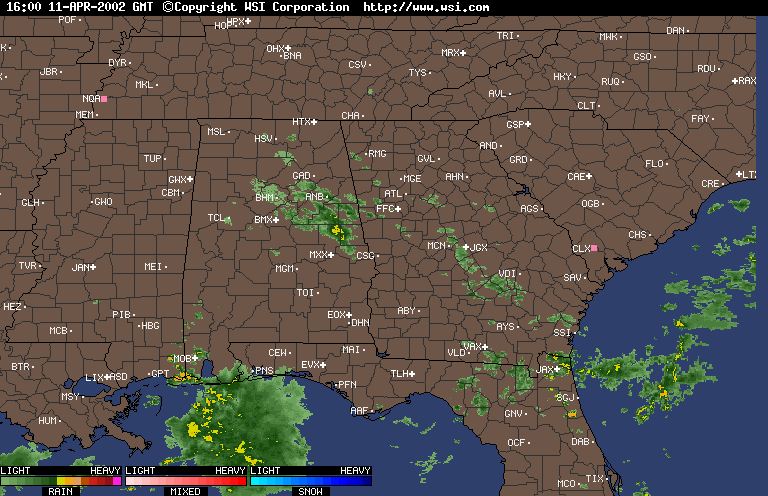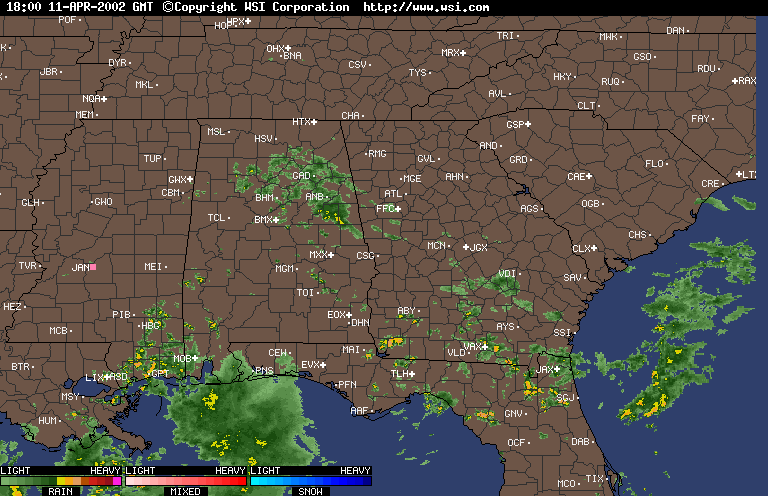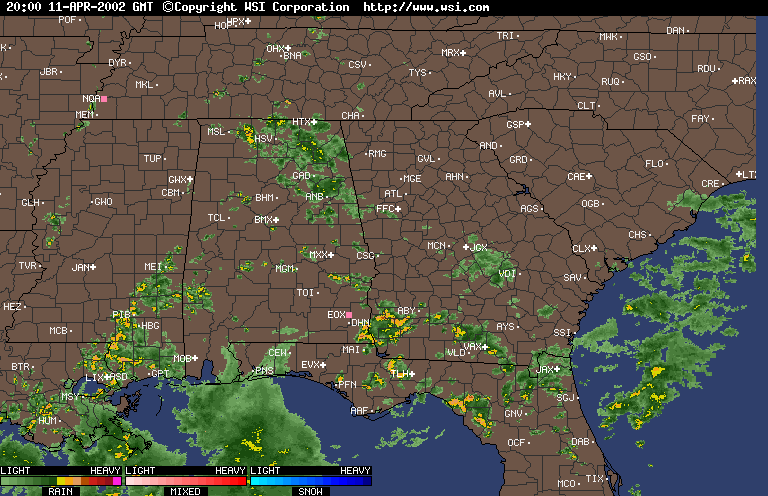
From what I have observed from the Satilette Photos and Radar is the chemical trails play a significant role in reducing precipitation over a given area. In my observations it takes about 3 hours for the clouds to fall apart completly. I believe that the air between the chemical trail and the precipiation producing clouds is being heated enough to prevent rain or snow fall.
Experiment:
In order to find out how significantly chemical trails play in destorying rainfall, I will devise a mock experiment in which chemical trails are laid just infinitelessmly above the maximum height of the cloud. This will give us the maximum amount of heating possible, as all the heat is directed to the cloud. The set up is as follows. There is a band of showers approaching a region. The clouds are 2000ft thick with the cloud base at 5000ft. The temperature is constant throughout the cloud, and the rising air can condensate into precipiation at the base of the cloud. We are assuming that the cloud remains at 100% throughout the entire 3 hours and the experiment is done during the day. The surface pressure is at 1013 mb. The Barium Salts are sprayed and a cirrus type cloud forms just above 7000ft. The rising air column remains constant through the 3 hours. The Goal of the experiment is to find out how much the extra radative solar forcing will heat up the air in the cloud. From that we can determine the water vapor needed to keep the cloud at 100% humidity, and then subtract that result from the extra water vapor from the rising air column. This will give us our rainfall reduction.
First, we need to calculate the amount of extra radative forcing that the chemical trail is causing in the cloud. We devise this by creating a simple greenhouse model, where the chemical trail has an albedo A* and the cloud has an albedo A0. The solar forcing comes into the cloud, and a portion of it is reflected back to space and another portion is reflected into the cloud. Then it is reflected back to the chemical trail, and a portion of that forcing is let through back into space, and the other part is reflected back to the cloud. This diagrams illustrates the procedure.
“We assume random spatial overlap between A* and A0. The total albedo At from the superimposed albedo layers A* and A0 is the sum of the fluxes of all radation beams reflected upward to space, divided by the incoming downward radation.”
Flux Fs:
We can simplify the albedo to : A= A0*(1-A*)^2
Using the equation:
Delta F = Fs*(1-A)^2 / 4
Where Fs= 1370 W/m^2 and Delta F is the change in flux.
Now we have an equation to determine the extra amount of heat radiated into the cloud. Next we need to determine the heat capacity of the air inside the cloud. First we need the amount of water in the cloud (g/m^3) before we can calculate the heat capacity of the air. We assume a constant T throughout the cloud.
nx= Px*Av / R*T
nx=number density, Av= Advagado’s Number, Px= partial pressure from water vapor
R= Universal constant, T=temperature
next we Substiute the number density into the partical density to determine grams/m^3 of water
px= nx* Mw / Av
px=density of water (g/m^3) in the cloud, Mw= molecular weight of water
Next we need to determine the amount of atmosphere between the base and top of the cloud. To solve this we need the results of the Barometric Law. We assume that the surface temperature is the same as the cloud.
Find the scale height.
H= R*T/ Ma* g
H= scale height, Ma= molcular weight of air(grams/mole) and g is gravity(m/s^2),
T is temperature
We substitute this into the barometric law.
P(z)= P(o) *exp^-(Z/H)
Where is P(z) is the pressure at the respective altitude. P(o) is surface pressure, Z is the altitude(meters), H=scale height.
To find the weight of the atmosphere we subtract the pressure at 5000ft, from the pressure at 7000ft.
Having this number, we can now calculate the heat capcity of the air.
First we must subtract the amount of water in the air, by multiplying the density by the 610 meters. Next we take that number and subtract it from the weight of the atmosphere between 5000 and 7000ft. Now we have the weights of both substances. We then must multiply each weight by its respective heat capcity. 1.003KJ/ kg*K for air and 1.870KJ/ kg *K for water vapor. Next we add those two number and divide by the total weight.
Now we have the combined heat capcity.
| Temperature | Cp |
| 16 C | 1.015 KJ/kg*K |
| 14 C | 1.012 KJ/kg*K |
| 10 C | 1.011 KJ/kg*K |
| 5 C | 1.009 KJ/kg*K |
| 0 C | 1.008 KJ/kg*K |
| 5 C | 1.006 KJ/kg*K |
Q=Fs W/m^2 * 3600 seconds/hr * 3 hours
Substituting Q into:
Delta T= Q /(m * cp)
| Delta F | Initial H20 Density | Initial H20 weight | Initial Temp | H Scale Height |
| 84.4 W/ m^2 | 13.51 g/m^3 | 8.277 kg | 16C | 8.46 km |
| 84.4 W/m^2 | 12.04 g/m^3 | 7.344 kg | 14C | 8.32 km |
| 84.4 W/m^2 | 9.40 g/m^3 | 5.734 kg | 10 C | 8.29 km |
| 84.4 W/m^2 | 6.79 g/m^3 | 4.142 kg | 5C | 8.14 km |
| 84.4 W/m^2 | 4.85 g/m^3 | 2.959 kg | 0C | 8.00 km |
| 84.4 W/m^2 | 3.25 g/m^3 | 1.982 kg | -5C | 7.85 km |
| Final Temp | Rain Lost | water vapor into cloud | % of rain lost |
| 17.5C | 0.616 kg | 4.4 kg | 14 % |
| 15.5C | 0.707 kg | 3.9 kg | 18% |
| 11.5C | 0.671 kg | 3.1 kg | 22% |
| 6.5C | 0.690 kg | 2.2 kg | 32% |
| 1.5C | 0.498 kg | 1.6 kg | 32% |
| -3.6C | 0.381 kg | 1.1 kg | 36% |
From the data, this is the absoulte maximum that chemical trails can do to a cloud. It appears that it is possible to break up fog with chemical trials, or a haze of stratus clouds. We can see that a range from 14 to 36 percent over three hours of the possible rain is lost if the chemical trail was directly on top of the impending clouds. Most Chemical trails are present above 10000ft, and thus the heat capacity of the air in between the clouds and the chemical trail would certainly absorb the heat, and make a temperature change minute, even smaller then 1.5K. There is an uncertainity with the albedo of the chemical trail, I assumed because it appears as a cirrus cloud to treat it as a cirrus cloud. Cirrus clouds typically have ranges of .3 to .5 for an albedo.
Conclusion
In a drought situation every bit of rainfall helps even if it is a tenth of an inch. Maybe there is something else that is going on between the cloud and the chemical trails that I cannnot observe. The information that was given to me is that possibly the Russians are changing the jet stream through a new weapon , scalar waves. The scalar waves, travel several times faster then the speed of light and thus they become a ripple in space time. If this is true then we have to re-teach the theory of relativity, because the speed limit of the universe under relativity is 3*10^8 m/s. Whatever is going on it is definitly man made. There is too much of a connection between the start of the current drought and the World Trade Center attacks. Someone is in a silent war with the United States.
References: